The Institute of Myology offers services both for basic research, for which it has set up a biological resource center, and for clinical research, for which it has platforms for conducting clinical trials in children and adults, and for cohort follow-up in particular.
- Myobank-AFM platform
The Institute of Myology’s Myobank-AFM biological resource centre is a not-for-profit service that collects, prepares, stores and distributes biological resources of human fluids and tissues. Its aim is to facilitate research into neuromuscular diseases.
Samples are taken mainly from people with neuromuscular or rare diseases, but also from healthy subjects, in order to provide a batch of “control” material. This biological material is intended for “in vitro” studies to supplement the knowledge acquired prior to “in vivo” clinical exploration, and is for research use only.
Myobank-AFM is certified to the Quality standard for biological resource centers. It is a member of the European biological material network, Eurobiobank, and is housed within the Plateforme Commune de Ressources Biologiques located on the Pitié-Salpêtrière Hospital site.
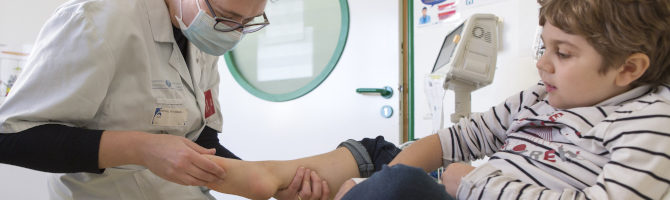
- I-Motion clinical trials platforms
The I-Motion platforms enable clinical trials to be conducted on adults (I-Motion Adults) and children (I-Motion Paediatrics) suffering from neuromuscular diseases, using specialised and dedicated expertise, a large active patient file, the support of all the departments at the Institute of Myology and privileged contacts within the hospital. They are the key point of contact for partners wishing to set up clinical trials in neuromuscular and/or neuropaediatric pathologies.
These platforms are structured in such a way as to provide integrated management of the various phases of research, from feasibility assessment to trial implementation and follow-up of protocol visits. Administrative and regulatory procedures before and during the trial are optimized and integrated into the operation of the platforms, allowing the investigator to devote full attention to patient recruitment and follow-up.
These two platforms are a guarantee of success in terms of recruitment and the timetable for trials, with high standards for the quality of trials, whether they are promoted by industry or academia.
- Registries et Databases Unit
The aim of the Registries and Databases unit is to develop and monitor databases, registries, observatories and cohort monitoring. The objective is to collect epidemiological and natural history data, assess the effect of treatments and prepare for future therapeutic trials.