Head: Anne Bigot
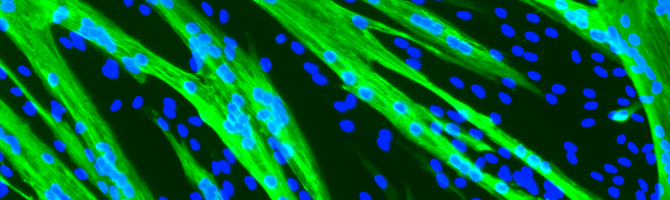
With the development of numerous innovative therapeutic approaches for genetic diseases, targeting DNA, RNA or proteins, muscle cells isolated from patients, known as myoblasts, represent an ideal in vitro model for evaluating these approaches for neuromuscular diseases. These cells have the advantage of being simple to use, reducing the number of animal experiments and also of carrying the patient’s exact mutation in its own genetic environment. However, there are limits to these in vitro approaches: human somatic cells have a limited capacity for proliferation, regulated by the mitotic clock, and reach replicative senescence after a defined number of divisions. This proliferation limit is reached even earlier in degenerative diseases. MyoLine neutralises replicative senescence in human muscle cells using double transduction with lentiviral vectors, creating immortal human cell lines.
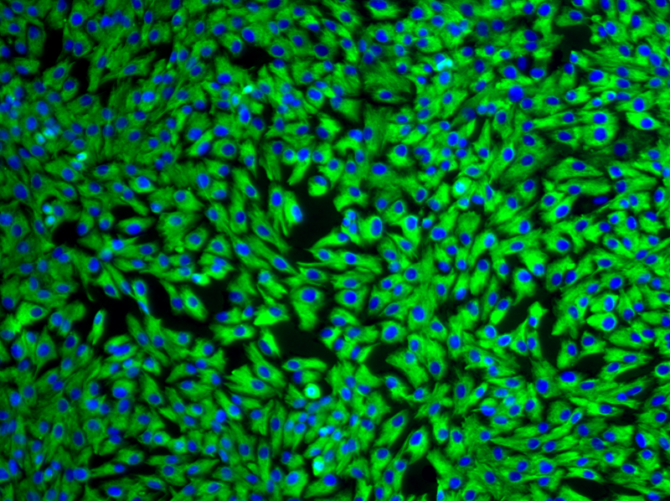
 Since its creation in 2007, the Institute of Myology’s human cell immortalisation platform has generated more than 200 human myoblast lines isolated from patients suffering from more than 36 different diseases (DMD, LGMD, FSHD, SMA, etc.). As access to muscle biopsies may be limited for certain diseases, we have also developed the immortalisation of skin fibroblasts. These immortalised fibroblasts are then transduced by an inducible transcription factor MyoD, and these myoconverted cells form myotubes and express muscle markers like muscle cells.
Since its creation in 2007, the Institute of Myology’s human cell immortalisation platform has generated more than 200 human myoblast lines isolated from patients suffering from more than 36 different diseases (DMD, LGMD, FSHD, SMA, etc.). As access to muscle biopsies may be limited for certain diseases, we have also developed the immortalisation of skin fibroblasts. These immortalised fibroblasts are then transduced by an inducible transcription factor MyoD, and these myoconverted cells form myotubes and express muscle markers like muscle cells.
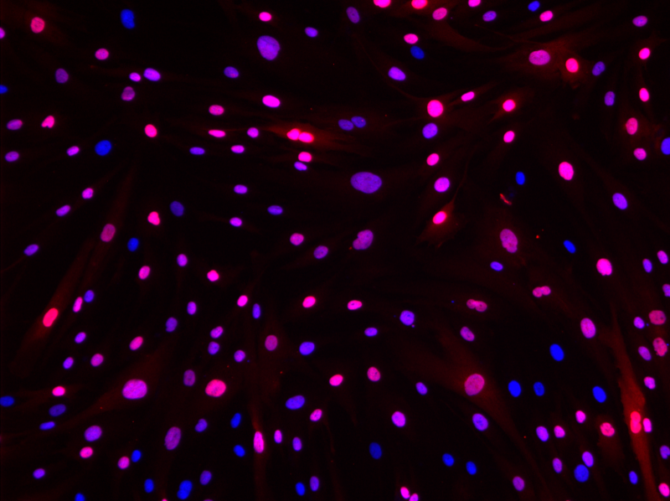
 The loss of muscle mass in patients with neuromuscular diseases is often accompanied by its replacement by fibrous and/or adipose tissue. In recent years, research in this field has focused on the role played by fibro-adipogenic progenitors (FAPs) because of their ability to differentiate into adipocytes or fibroblastic cells. To support this research, MyoLine is currently developing models of FAPs isolated from various muscular dystrophies.
The loss of muscle mass in patients with neuromuscular diseases is often accompanied by its replacement by fibrous and/or adipose tissue. In recent years, research in this field has focused on the role played by fibro-adipogenic progenitors (FAPs) because of their ability to differentiate into adipocytes or fibroblastic cells. To support this research, MyoLine is currently developing models of FAPs isolated from various muscular dystrophies.
For several years, these cell lines have been made available to the scientific community on a collaborative basis, and many international laboratories are already using them. Where consent signed by the donor permits, cell lines can also be used under material transfer agreements for the development of therapeutic tools by private partners.
Please send your requests to anne.bigot@sorbonne-universite.fr & vincent.mouly@sorbonne-universite.fr

Team members
Bigot Anne, Research Project Leader, Platform Manager
Bensalah Mona, Engineer
Diarra Rokiatou, Apprentice
Filachet Alexis, Apprentice
Mamchaoui Kamel, Engineer
Mouly Vincent, Research Director Emeritus
Ohana Jessica, Engineer
Publications 2024
- Bin Haidar H, Almeida JR, Williams J, Guo B, Bigot A, Senthilkumaran S, Vaiyapuri S, Patel K. Differential effects of the venoms of Russell’s viper and Indian cobra on human myoblasts. Sci Rep. 2024 Feb 7;14(1):3184. doi: 10.1038/s41598-024-53366-9. PMID: 38326450 Free PMC article.
- Lemmers RJLF, Butterfield R, van der Vliet PJ, de Bleecker JL, van der Pol L, Dunn DM, Erasmus CE, D’Hooghe M, Verhoeven K, Balog J, Bigot A, van Engelen B, Statland J, Bugiardini E, van der Stoep N, Evangelista T, Marini-Bettolo C, van den Bergh P, Tawil R, Voermans NC, Vissing J, Weiss RB, van der Maarel SM. Autosomal dominant in cis D4Z4 repeat array duplication alleles in facioscapulohumeral dystrophy. Brain. 2024 Feb 1;147(2):414-426. doi: 10.1093/brain/awad312. PMID: 37703328 Free PMC article.
- De Spiegeleer A, Descamps A, Wynendaele E, Naumovski P, Crombez L, Planas M, Feliu L, Knappe D, Mouly V, Bigot A, Bielza R, Hoffmann R, Van Den Noortgate N, Elewaut D, De Spiegeleer B. Streptococcal quorum sensing peptide CSP-7 contributes to muscle inflammation and wasting. Biochim Biophys Acta Mol Basis Dis. 2024 Apr;1870(4):167094. doi: 10.1016/j.bbadis.2024.167094. Epub 2024 Feb 29. PMID: 38428683
- Galli F, Bragg L, Rossi M, Proietti D, Perani L, Bacigaluppi M, Tonlorenzi R, Sibanda T, Caffarini M, Talapatra A, Santoleri S, Meregalli M, Bano-Otalora B, Bigot A, Bozzoni I, Bonini C, Mouly V, Torrente Y, Cossu G. Cell-mediated exon skipping normalizes dystrophin expression and muscle function in a new mouse model of Duchenne Muscular Dystrophy. EMBO Mol Med. 2024 Apr;16(4):927-944. doi: 10.1038/s44321-024-00031-3. Epub 2024 Mar 4. PMID: 38438561 Free PMC article.
- Yin A, Fu W, Elengickal A, Kim J, Liu Y, Bigot A, Mamchaoui K, Call JA, Yin H. Chronic hypoxia impairs skeletal muscle repair via HIF-2α stabilization. J Cachexia Sarcopenia Muscle. 2024 Apr;15(2):631-645. doi: 10.1002/jcsm.13436. Epub 2024 Feb 9. PMID: 38333911 Free PMC article.
- Handal T, Juster S, Abu Diab M, Yanovsky-Dagan S, Zahdeh F, Aviel U, Sarel-Gallily R, Michael S, Bnaya E, Sebban S, Buganim Y, Drier Y, Mouly V, Kubicek S, van den Broek WJAA, Wansink DG, Epsztejn-Litman S, Eiges R. Differentiation shifts from a reversible to an irreversible heterochromatin state at the DM1 locus. Nat Commun. 2024 Apr 16;15(1):3270. doi: 10.1038/s41467-024-47217-4. PMID: 38627364 Free PMC article.
- Granados A, Zamperoni M, Rapone R, Moulin M, Boyarchuk E, Bouyioukos C, Del Maestro L, Joliot V, Negroni E, Mohamed M, Piquet S, Bigot A, Le Grand F, Albini S, Ait-Si-Ali S. SETDB1 modulates the TGFβ response in Duchenne muscular dystrophy myotubes. Sci Adv. 2024 May 3;10(18):eadj8042. doi: 10.1126/sciadv.adj8042. Epub 2024 May 1. PMID: 38691608 Free PMC article.
- Núñez-Manchón J, Capó J, Martínez-Piñeiro A, Juanola E, Pesovic J, Mosqueira-Martín L, González-Imaz K, Maestre-Mora P, Odria R, Savic-Pavicevic D, Vallejo-Illarramendi A, Mamchaoui K, Bigot A, Mouly V, Suelves M, Nogales-Gadea G. Immortalized human myotonic dystrophy type 1 muscle cell lines to address patient heterogeneity. iScience. 2024 May 7;27(6):109930. doi: 10.1016/j.isci.2024.109930. eCollection 2024 Jun 21. PMID: 38832025 Free PMC article.
- Engal E, Sharma A, Aviel U, Taqatqa N, Juster S, Jaffe-Herman S, Bentata M, Geminder O, Gershon A, Lewis R, Kay G, Hecht M, Epsztejn-Litman S, Gotkine M, Mouly V, Eiges R, Salton M, Drier Y. DNMT3B splicing dysregulation mediated by SMCHD1 loss contributes to DUX4 overexpression and FSHD pathogenesis. Sci Adv. 2024 May 31;10(22):eadn7732. doi: 10.1126/sciadv.adn7732. Epub 2024 May 29. PMID: 38809976 Free PMC article.
- Moreno N, Sabater-Arcis M, Sevilla T, Alonso MP, Ohana J, Bargiela A, Artero R. Therapeutic potential of oleic acid supplementation in myotonic dystrophy muscle cell models. Biol Res. 2024 May 17;57(1):29. doi: 10.1186/s40659-024-00496-z. PMID: 38760841 Free PMC article.
- Ghosh S, Arshi MU, Ghosh S, Jash M, Sen S, Mamchaoui K, Bhattacharyya S, Rana NK, Ghosh S. –Discovery of Quinazoline and Quinoline-Based Small Molecules as Utrophin Upregulators via AhR Antagonism for the Treatment of Duchenne Muscular Dystrophy. J Med Chem. 2024 Jun 13;67(11):9260-9276. doi: 10.1021/acs.jmedchem.4c00398. Epub 2024 May 21. PMID: 38771158
- Beaufils M, Melka M, Brocard J, Benoit C, Debbah N, Mamchaoui K, Romero NB, Dalmas-Laurent AF, Quijano-Roy S, Fauré J, Rendu J, Marty I. Functional benefit of CRISPR-Cas9-induced allele deletion for RYR1 dominant mutation. Mol Ther Nucleic Acids. 2024 Jun 17;35(3):102259. doi: 10.1016/j.omtn.2024.102259. eCollection 2024 Sep 10. PMID: 39071953 Free PMC article.
- Rich J, Bennaroch M, Notel L, Patalakh P, Alberola J, Issa F, Opolon P, Bawa O, Rondof W, Marchais A, Dessen P, Meurice G, Le-Gall M, Polrot M, Ser-Le Roux K, Mamchaoui K, Droin N, Raslova H, Maire P, Geoerger B, Pirozhkova I. DiPRO1 distinctly reprograms muscle and mesenchymal cancer cells. EMBO Mol Med. 2024 Aug;16(8):1840-1885. doi: 10.1038/s44321-024-00097-z. Epub 2024 Jul 15. PMID: 39009887 Free PMC article.
- Grandi FC, Astord S, Pezet S, Gidaja E, Mazzucchi S, Chapart M, Vasseur S, Mamchaoui K, Smeriglio P. Characterization of SMA type II skeletal muscle from treated patients shows OXPHOS deficiency and denervation. JCI Insight. 2024 Sep 12;9(20):e180992. doi: 10.1172/jci.insight.180992. PMID: 39264856 Free PMC article.
- Lemoine J, Dubois A, Dorval A, Jaber A, Warthi G, Mamchaoui K, Wang T, Corre G, Bovolenta M, Richard I. Correction of exon 2, exon 2-9 and exons 8-9 duplications in DMD patient myogenic cells by a single CRISPR/Cas9 system. Sci Rep. 2024 Sep 11;14(1):21238. doi: 10.1038/s41598-024-70075-5. PMID: 39261505 Free PMC article.
- Poyatos-García J, Soblechero-Martín P, Liquori A, López-Martínez A, Maestre P, González-Romero E, Vázquez-Manrique RP, Muelas N, García-García G, Ohana J, Arechavala-Gomeza V, Vílchez JJ. Deletion of exons 45 to 55 in the DMD gene: from the therapeutic perspective to the in vitro model. Skelet Muscle. 2024 Oct 1;14(1):21. doi: 10.1186/s13395-024-00353-3. PMID: 39354597 Free PMC article.
- Heaton RA, Ball ST, Staunton CA, Mouly V, Jones SW, McArdle A, Jackson MJ. Peroxiredoxin 2 mediates redox-stimulated adaptations to oxidative phosphorylation induced by contractile activity in human skeletal muscle myotubes. Free Radic Biol Med. 2024 Dec 4;227:395-406. doi: 10.1016/j.freeradbiomed.2024.11.053. Online ahead of print. PMID: 39643135 Free article.
- Anwar S, Roshmi RR, Woo S, Haque US, Arthur Lee JJ, Duddy WJ, Bigot A, Maruyama R, Yokota T. Antisense oligonucleotide-mediated exon 27 skipping restores dysferlin function in dysferlinopathy patient-derived muscle cells. Mol Ther Nucleic Acids. 2024 Dec 21;36(1):102443. doi: 10.1016/j.omtn.2024.102443.