Eline Lemerle completed her thesis* under the supervision of Stéphane Vassilopoulos, in Marc Bitoun’s team (Muscle Organization & Therapy of Dominant Centronuclear Myopathy) at the Institute’s Myology Centre for Research. Her work on the formation of T-tubules in the muscle cell and more specifically on the role of caveolae, has just been published in the journal eLife**. Interview with Eline Lemerle and Stéphane Vassilopoulos.
What is the background to this work?
T-tubules are invaginations of the sarcolemma and constitute a highly specialised compartment of the muscle cell. Mutations in proteins involved in the formation or function of these T-tubules are associated with many congenital myopathies and muscular dystrophies, and even beyond that, T-tubule defects and dysfunctions are indirectly involved in other muscular dystrophies.
The T-tubule surrounded by two terminal cisternae of the sarcoplasmic reticulum form a triad, a highly specialised membrane structure required for the excitation-contraction coupling characteristic of the muscle cell. While we know the elements that form the triad (Bin1***, caveolae, Ryr1 calcium channels, …), we have not yet understood when, how or by what mechanism they are formed. The only thing we know is that the accumulation of caveolae at the plasma membrane is the initial step in the formation of T-tubules, and that at some point Bin1 comes into play and gives the membrane its tubular shape. But, for example, the time at which the sarcoplasmic reticulum connects with the tubules is not known.
What was the objective of this study and what results did you obtain?
Our objective was to revisit the mechanism of T-tubule formation in the muscle cell and in particular the role of caveolae.
To do this, we had to find new experiments and change the way we looked at things in order to produce new knowledge. To do this we examined the nanoscale composition of nascent T-tubules using a correlative microscopy technique combining super-resolution fluorescence, fluorescence microscopy and electron microscopy on platinum replicas (PREM). These techniques were applied to control and genetically modified human and murine skeletal muscle myoblasts differentiated into multinucleated myotubes long enough for these structures to form.
What conclusions did you draw?
Our results show that, at the plasma membrane, Bin1 and caveolae composed of caveolin-3 (Cav3) assemble into ring structures from which tubes emerge. Expression of Bin1 leads to the formation of both rings and tubes. We have shown that Bin1 forms scaffolds on which caveolae accumulate to form the initial T-tubule. Cav3 deficiency caused by gene silencing or pathogenic mutations generates both defective ring formation and Bin1-mediated tubulation disorganisation.
This work has enabled us to identify new mechanisms that may be useful to better understand the pathophysiology of myopathies caused by Cav3 or Bin1 dysfunction and will allow us to better target therapeutic strategies for several different myopathies that share T-tubule defects.
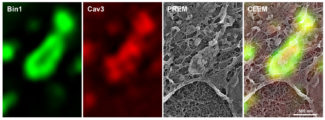
Fig.1: Immunofluorescent labelling of a caveolae ring in a murine myotube transduced with Bin1-GFP (green) and labelled with antibodies against Cav3 (red) observed by super-resolution light microscopy. The last image on the right shows the overlay (correlative microscopy) of the super-resolution images with the ultrastructure image obtained by platinum replica electron microscopy (PREM).
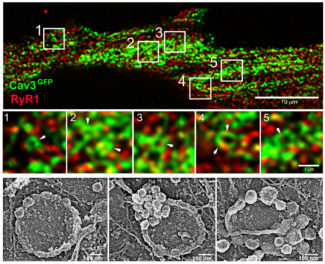
Fig.2: (Top) Super-resolution images of a human myotube expressing Cav3-GFP unroofed by ultrasounds and labelled with antibodies against the RyR1 calcium channel (red). Cav3-positive rings are indicated by white arrowheads.
(Bottom) Platinum replica electron microscopy image gallery of caveolae rings in human myotubes.
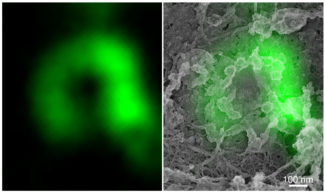
Fig.3: (Left) Super-resolution fluorescence microscopy image of a Cav3-labelled ring.
(Right) Overlay of Cav3 fluorescent labelling with the PREM electron microscopy image of the same ring.
* “Role of caveolae in T-tubule formation and in the pathophysiology of caveolinopathies”
*** Bin1 is mutated in the recessive form of centronuclear myopathy. Defects in the splicing of exon 11 (the one that allows tubulation) were discovered in DM1 by Denis Furling’s team (Fugier et al., Nat Medecine)