Find below all the job offers from the Institute of Myology.
PhD opportunity BioMaps - RMN Lab, Center of Research in Myology
PhD opportunity at the Nuclear Magnetic Resonance Laboratory at Institute of Myology.
Low Field MR-Fingerprinting for respiratory assessment
Keywords: Low-field MRI, sequence development, undersampled reconstruction and optimization, AI-based denoising, respiratory function.
➣ Context
Pulmonary and muscle tissues involved in the respiratory function can be affected in various diseases (COPD, neuromuscular diseases, CoViD…). Quantitative MRI has become a key tool for studying neuromuscular diseases(1), but it mainly focuses on static limb muscles. Imaging the lungs and respiratory muscles is challenging due to motion and low MR signal levels. Current diagnostic tools have limitations, such as low sensitivity or radiation exposure. In this context, having access to tissue composition (fat fraction, vascularization…) on top of functional measures would be of great interest but is particularly challenging.
This PhD project aims at exploring jointly quantitative MRI and low-field MRI. Quantitative MRI provides valuable biomarkers, but requires long acquisition, while low-field MRI offers better accessibility, improved contrast and reduced field inhomogeneities(2), making it ideal for thoracic imaging. Nevertheless, the reduced signal-to-noise ratio is still a challenge in low-field MRI.
➣ Framework
Magnetic Resonance Fingerprinting (MRF) is a new method(3) designed to overcome these challenges. By varying acquisition parameters, MRF generates a unique signal “fingerprint” for each tissue, enabling simultaneous extraction of multiple quantitative parameters through pattern recognition. Since MRF is less sensitive to noise, it is particularly promising for low-field MRI, potentially improving respiratory imaging(4). An MRF sequence(5), compensating the respiratory motion MoCo MRF T1-FF (6) has been designed and validated at the Institute of Myology for the characterization of muscle tissues by measuring 5 parameters simultaneously(7), on a 3T MRI (Siemens Healthineers). These developments will be the basis for a novel MRF sequence sensitive to additional parameters of interest (T2, water T2, vascularization) and adapted to the low-field constraints.
The validation on low field acquisitions will be done at the BioMaps laboratory, which recently acquired a 0.55 T MRI (Siemens Healthineers). To pave for the low SNR at 0.55 T, existing iterative reconstruction algorithms implemented at the Institute of Myology will be leveraged.
A 3D magnetic resonance spirometry sequence Spiro3D (8) was developed at the Biomaps laboratory providing a rich set of 3-dimensional parameters characterizing respiratory function and mechanics spatially. It was validated on healthy subjects and is currently being adapted to the 0.55 T scanner.
Both sequences are already running on clinical sites at 1.5T and 3T. The quantitative tissue measurements obtained by MRF will be compared against the functional measurements allowed by Spiro 3D on a small cohort of healthy subjects and patients.
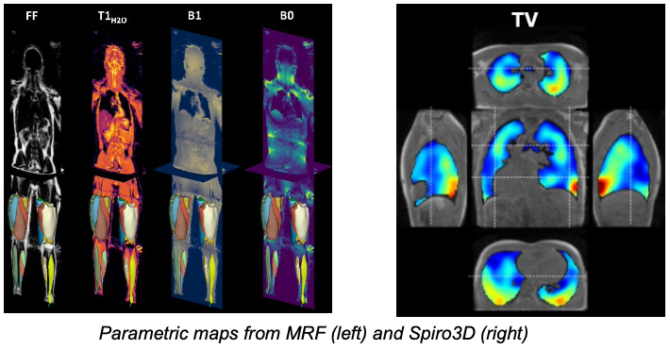
➣ Objectives
The objective of this PhD project is to develop, optimize, and test 3D MR Fingerprinting techniques to quantify key parameters for the lungs and respiratory muscles. The PhD candidate will contribute to the following advancements:
- Development of MR Fingerprinting sequences sensitive to key respiratory parameters
- Optimization framework for MR Fingerprinting sequences under low-field constraints
using digital twins - Reconstruction of denoised 3D quantitative maps
- Validation of quantitative measurements against functional assessments in-vivo on healthy subjects and patients
➣ Environment
This PhD work will be split between the sites of BioMaps and Institute of Myology, in collaboration with Siemens Healthineers. It will be directed by Benjamin Marty (Co-head of the NMR laboratory, Institute of Myology), and co-supervised by Constantin Slioussarenko (Senior Researcher, Institute of Myology) and Angéline Nemeth (Associate Professor, Biomaps, Paris- Saclay University). The ongoing collaboration with Siemens on both sites will be leveraged, with a potential partnership on this specific project.
During the thesis, the PhD candidate will have access to two MRI systems installed inside the two laboratories: 0.55 T MRI (Biomaps) and 3T MRI (Institute of Myology). Collaborations are already planned with the thoracic radiology services of Raymond-Poincaré and Pitié- Salpêtrière AP-HP hospitals and their 1.5 T MRI.
The future PhD candidate will be in close interaction with current PhD candidates from the European V|LF-Spiro3D project led by BioMaps. This project brings together eleven partners (laboratories, hospitals, industrial companies) from four European countries for the development of 3D MRI lung function assessment. A collaboration with the AMT Center, University of Aberdeen, specialized in fingerprinting in very low-field MRI, is also being explored.
➣ CandidateProfile
- Engineering degree or Master’s (M2) specializing in applied mathematics, physics, medical imaging, or related fields.
- Excellent programming skills, particularly in Python. C++ programming experience is a plus.
- Ideally, experience in AI/ML (training/validation).
- Scientific curiosity, interest in experimentation.
- Strong proficiency in English (C1 level).
➣ Contact
Benjamin Marty: b.marty@institut-myologie.org
Angéline Nemeth: angeline.nemeth@universite-paris-saclay.fr
Constantin Slioussarenko: c.slioussarenko@institut-myologie.org
Bibliography
- (1) Marty, B.; Baudin, P. Y.; Reyngoudt, H.; Azzabou, N.; Caldas de Almeida Araújo, E.; Carlier, P. G.; Loureiro de Sousa, P. Simultaneous Muscle Water T2 and Fat Fraction Mapping Using Transverse Relaxometry with Stimulated Echo Compensation. NMR Biomed. 2016, 29 (4), 431–443. https://doi.org/10.1002/nbm.3459.
- (2) Sarracanie, M.; Salameh, N. Low-Field MRI: How Low Can We Go? A Fresh View on an Old Debate. Front. Phys. 2020, 8, 172. https://doi.org/10.3389/fphy.2020.00172.
- (3) Ma, D.; Gulani, V.; Seiberlich, N.; Liu, K.; Sunshine, J. L.; Duerk, J. L.; Griswold, M. A. Magnetic Resonance Fingerprinting. Nature 2013, 495 (7440), 187–192. https://doi.org/10.1038/nature11971.
- (4) Liu, Z.; Lugogo, N.; Agarwal, P.; Hamilton, J. Feasibility of Lung MR Fingerprinting at 0.55T Using a Deep Image Prior Reconstruction; Toronto, ON, Canada; p 2675. https://doi.org/10.58530/2024/2675.
- (5) Slioussarenko, C.; Baudin, P.; Marty, B. A Steady‐state MR Fingerprinting Sequence Optimization Framework Applied to the Fast 3D Quantification of Fat Fraction and Water T1 in the Thigh Muscles. Magn. Reson. Med. 2025, mrm.30490. https://doi.org/10.1002/mrm.30490.
- (6) Slioussarenko, C.; Baudin, P.-Y.; Lapert, M.; Marty, B. Upper-Body Free-Breathing Magnetic Resonance Fingerprinting Applied to the Quantification of Water T1 and Fat Fraction. arXiv September 24, 2024. http://arxiv.org/abs/2409.16200 (accessed 2024-09-25).
- (7) Slioussarenko, C. Whole-Body Quantitative Imaging of the Skeletal Muscle by Magnetic Resonance Fingerprinting. Theses, Université Paris-Saclay, 2024. https://theses.hal.science/tel-04939151.
- (8) Barrau, N. 3D MR Spirometry. Theses, Université Paris-Saclay, 2024. https://theses.hal.science/tel- 04663088.
Download the complete announcement of the PhD opportunity at the Nuclear Magnetic Resonance Laboratory at Institute of Myology : Low Field MR-Fingerprinting for respiratory assessment
PhD opportunity GenoTher - RMN Lab, Centrer in Research in Myology
PhD opportunity at the Nuclear Magnetic Resonance Laboratory at Institute of Myology.
Development of real-time quantitative MRI for muscles involved in respiration, swallowing and eye movements
Keywords: Muscle MRI, real-time MRI, undersampled reconstruction, non-Cartesian MRI acquisition
➣ Context
Quantitative MRI (qMRI) is a well-established tool for the in vivo characterization and longitudinal monitoring of skeletal muscle pathology in neuromuscular diseases(1). It is increasingly used in clinical trials, particularly targeting appendicular muscles (limbs) through static imaging protocols such as T1-weighted, Dixon, and T2 mapping(2).
However, these conventional methods are limited in their ability to assess small, continuously active muscles involved in essential functions like respiration, swallowing, and eye movement—muscles that are often impaired in neuromuscular conditions and play a significant role in patient morbidity and mortality(3,4). These muscle groups remain underexplored due to challenges in imaging dynamic motion and achieving sufficient spatial resolution.
To address this gap, real-time MRI has emerged as a promising solution, enabling high- temporal-resolution imaging via rapid, undersampled acquisitions(5). Reconstruction techniques leverage either compressed sensing with temporal-spatial regularization(6) or deep learning approaches, which improve reconstruction fidelity and speed(7,8).
Originally applied in domains like cardiac cine imaging(9), real-time MRI offers a valuable platform for capturing the functional dynamics of non-limb muscles, with potential to deliver novel biomarkers for both disease progression and therapeutic efficacy assessment.
➣ Framework
A 3D radial MRI sequence (MoCo MRF T1-FF(10)) compatible with real-time imaging, along with its reconstruction pipeline, has been implemented at the NMR laboratory of the Institute of Myology and validated on a 3T MRI system (Siemens Healthineers). This development will serve as the foundation for a new real-time MRI sequence targeting respiratory muscles. In parallel, quantitative MRI protocols have been established to assess fat fraction and inflammation in both respiratory(10) and tongue muscles(11), enabling correlation between structural tissue biomarkers and functional metrics from real-time imaging. Additionally, in- house segmentation algorithms (museg-ai) could be adapted for dynamic tracking of muscles in motion. Functional assessments, such as tongue strength measurements, have been developed in collaboration with the laboratory of physiology and neuromuscular evaluation at the Institute of Myology, providing complementary data for validating MRI- derived functional biomarkers. 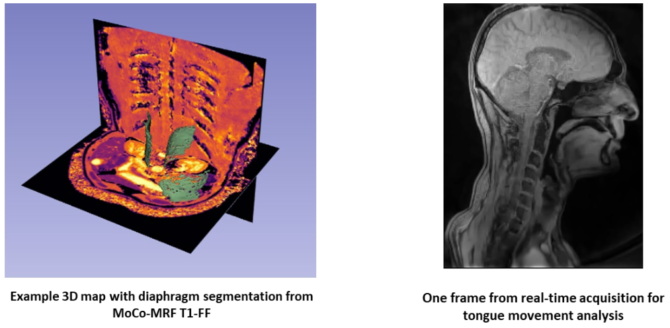
➣ Objectives
The objective of this thesis is to develop and optimize real-time MRI techniques in the context of quantitative imaging of dynamic skeletal muscles. The doctoral candidate will contribute to the following advancements:
- Real-time imaging and tracking of dynamic processes: Optimize acquisition sequences to capture and analyze the dynamics of muscles involved in respiration, swallowing, and eye movements.
- Optimization of 3D reconstructions: Advanced iterative reconstruction algorithms taking into account motion.
- Adaptation of segmentation algorithms for dynamic tracking of moving structures
- Development of functional biomarkers: Identify and quantify new biomarkers describing these processes to improve the characterization of functional alterations related to neuromuscular pathologies.
➣ Environment
This PhD work will be carried at the Institute of Myology. It will be directed by Benjamin Marty (Co-head of the NMR laboratory, Institute of Myology), and co-supervised by Constantin Slioussarenko (Senior Researcher, Institute of Myology). The ongoing collaboration with Siemens Healthineers will be leveraged, with one on-site clinical scientist.
During the thesis, the PhD candidate will have access to the 3T MRI system (PrismaFit Siemens Healthineers) installed at Institute of Myology. Existing collaborations with the radiology services of Raymond-Poincaré and Pitié-Salpêtrière AP-HP hospitals and their 1.5 T MRI could be leveraged as well for clinical applications. Biomechanical modelling with inputs from specialist laboratories are potential routes of investigation for developing biomarkers linked to the physiology.
The future PhD candidate will be in close interaction with current PhD candidates at Institute of Myology working on quantitative MRI and deep-learning muscles segmentation.
The funding for this PhD project is derived from the GenoTher biocluster project, part of the French Innovation Santé 2030 plan.
➣ CandidateProfile
- Engineering degree or Master’s (M2) specializing in applied mathematics, physics, medical imaging, or related fields.
- Excellent programming skills, particularly in Python. C++ programming experience is a plus.
- Ideally, experience in AI/ML (training/validation).
- Scientific curiosity, interest in experimentation.
- Strong proficiency in English (C1 level).
➣ Contact
Benjamin Marty: b.marty@institut-myologie.org
Constantin Slioussarenko: c.slioussarenko@institut-myologie.org
Bibliography
- (1) Marty, B.; Baudin, P. Y.; Reyngoudt, H.; Azzabou, N.; Caldas de Almeida Araújo, E.; Carlier, P. G.; Loureiro de Sousa, P. Simultaneous Muscle Water T2 and Fat Fraction Mapping Using Transverse Relaxometry with Stimulated Echo Compensation. NMR Biomed. 2016, 29 (4), 431–443. https://doi.org/10.1002/nbm.3459.
- (2) Carlier, P. G.; Marty, B.; Scheidegger, O.; Loureiro de Sousa, P.; Baudin, P.-Y.; Snezhko, E.; Vlodavets, D. Skeletal Muscle Quantitative Nuclear Magnetic Resonance Imaging and Spectroscopy as an Outcome Measure for Clinical Trials. J. Neuromuscul. Dis. 2016, 3 (1), 1–28. https://doi.org/10.3233/JND-160145.
- (3) Bourke, S. C. Respiratory Involvement in Neuromuscular Disease. Clin. Med. 2014, 14 (1), 72–75. https://doi.org/10.7861/clinmedicine.14-1-72.
- (4) Argov, Z.; De Visser, M. Dysphagia in Adult Myopathies. Neuromuscul. Disord. 2021, 31 (1), 5–20. https://doi.org/10.1016/j.nmd.2020.11.001.
- (5) Uecker, M.; Zhang, S.; Voit, D.; Karaus, A.; Merboldt, K.; Frahm, J. Real‐time MRI at a Resolution of 20 Ms. NMR Biomed. 2010, 23 (8), 986–994. https://doi.org/10.1002/nbm.1585.
- (6) Feng, L.; Axel, L.; Chandarana, H.; Block, K. T.; Sodickson, D. K.; Otazo, R. XD-GRASP: Golden-Angle Radial MRI with Reconstruction of Extra Motion-State Dimensions Using Compressed Sensing. Magn. Reson. Med. 2016, 75 (2), 775–788. https://doi.org/10.1002/mrm.25665.
- (7) He, Z.; Zhu, Y.-N.; Chen, Y.; Chen, Y.; He, Y.; Sun, Y.; Wang, T.; Zhang, C.; Sun, B.; Yan, F.; Zhang, X.; Sun, Q.-F.; Yang, G.-Z.; Feng, Y. A Deep Unrolled Neural Network for Real-Time MRI-Guided Brain Intervention. Nat. Commun. 2023, 14 (1), 8257. https://doi.org/10.1038/s41467-023-43966-w.
- (8) Ahmad, R.; Bouman, C. A.; Buzzard, G. T.; Chan, S.; Liu, S.; Reehorst, E. T.; Schniter, P. Plug-and-Play Methods for Magnetic Resonance Imaging: Using Denoisers for Image Recovery. IEEE Signal Process. Mag. 2020, 37 (1), 105–116. https://doi.org/10.1109/MSP.2019.2949470.
- (9) Longère, B.; Abassebay, N.; Gkizas, C.; Hennicaux, J.; Simeone, A.; Rodriguez Musso, A.; Carpentier, P.; Coisne, A.; Pang, J.; Schmidt, M.; Toupin, S.; Montaigne, D.; Pontana, F. A New Compressed Sensing Cine Cardiac MRI Sequence with Free-Breathing Real-Time Acquisition and Fully Automated Motion-Correction: A Comprehensive Evaluation. Diagn. Interv. Imaging 2023, 104 (11), 538–546. https://doi.org/10.1016/j.diii.2023.06.005.
- (10) Slioussarenko, C.; Baudin, P.-Y.; Lapert, M.; Marty, B. Upper-Body Free-Breathing Magnetic Resonance Fingerprinting Applied to the Quantification of Water T1 and Fat Fraction. arXiv September 24, 2024. http://arxiv.org/abs/2409.16200 (accessed 2024-09-25).
- (11) Vermeulen, E.; Baudin, P.-Y.; Lapert, M.; Marty, B. Quantitative Assessment of Tongue Tissue Structure with 3D Partially Spoiled Gradient Echo; Toronto, ON, Canada; p 0902. https://doi.org/10.58530/2024/0902.
Download the complete announcement of the PhD opportunity at the Nuclear Magnetic Resonance Laboratory at Institute of Myology: Development of real-time quantitative MRI for muscles involved in respiration, swallowing and eye movements
Two PhD positions - REDs Lab, Myology Research Center
ENTRY-DM: MSCA Doctoral network
Interdisciplinary doctoral training on oligonucleotide-based therapies for myotonic dystrophy
Two PhD positions are available from September 2025, in the laboratory Repeat Expansions and Myotonic Dystrophy, at the Myology Research Centre / Institute of Myology in Paris, France. The project is funded by a MSCA Doctoral network, coordinated by Mario Gomes-Pereira.
- NETWORK DESCRIPTION
ENTRY-DM is an interdisciplinary training and research programme focused on RNA-based therapeutics for myotonic dystrophy (DM). It offers 14 fully funded positions across top European institutions, integrating fundamental science, translational medicine, and clinical applications. The network integrates academic leaders, biotech experts, and patient advocates to develop disease models, optimise antisense oligonucleotide (ASO) therapies, and identify clinical biomarkers. Doctoral candidates will receive advanced training in genomics, bioinformatics, stem cell research, bioengineering, and neuropsychology. With host institutions in France, Spain, Italy, the Netherlands, Germany, and Poland, ENTRY-DM provides exceptional mobility, cross-sector training, and world-class supervision. It will equip doctoral candidates with the expertise to drive future breakthroughs in RNA therapeutics for rare disease treatment.
- PROJECT 1: Development of circulating muscle-specific biomarkers of myotonic dystrophy
This project aims to identify circulating biomarkers of DM1 muscle disease severity and treatment response by analysing extracellular vesicles in blood. Using DM1 cell models (human and mouse-derived) and isogenic controls, transcriptomic and proteomic analyses will be conducted to detect muscle-specific biomarkers. These will be validated in patient blood samples and assessed for responsiveness to various gene therapeutic approaches.
Supervisors: Genevieve Gourdon (genevieve.gourdon@inserm.fr); Denis Furling (denis.furling@sorbonne-universite.fr)
- PROJECT 2: Circulating biomarkers of brain dysfunction in myotonic dystrophy type 1
This project aims to identify brain-specific biomarkers to monitor disease progression and therapeutic response in DM1. The study will focus on the analysis of the secreted proteome, transcriptome and metabolome of neurons and astrocytes to reveal disrupted neuroglial interations and uncover candidate biomarkers of brain disease. Finally, we will evaluate how the neuronal and glial secretome responds to therapeutic interventions, ASOs and RNA-binding protein decoys, to support the development of future clinical monitoring tools.
Supervisor: Mario Gomes-Pereira (mario.pereira@inserm.fr)
- CANDIDATES PROFILE
We are looking for highly motivated and ambitious doctoral candidates, with a strong knowledge in molecular and cellular biology. A keen interest in muscle physiology or neurobiology, RNA-based therapeutics and biomarker discovery is essential. Applicants must hold an MSc degree in Life Sciences, Biomedical Sciences, or in a related field. Practical experience in molecular biology techniques (such as PCR, RT- PCR) and cell culture is required. The applicant must have not resided in France for more than 12 months in the 3 years immediately prior to recruitment.
Starting Date: September 2025
Application deadline: 30 May 2025
FOR FURTHER DETAILS, FULL ELIGIBILITY CRITERIA AND HOW TO APPLY:
👉 Project 1: https://euraxess.ec.europa.eu/jobs/324426
👉 Project 2: https://euraxess.ec.europa.eu/jobs/324434
Download the announcement of the two PhD positions
