
Charles Frison-Roche has received the Oral Research Communication Prize, awarded by the French Myology Society (SFM). He is a PhD student in the REDs team headed by Denis Furling and Geneviève Gourdon, at the Myology Research Centre and the Institute of Myology. Under the supervision of Frédérique Rau, he is working on the pathophysiology of myotonic dystrophy type 1 (DM1), a disease caused by a CTG repeat expansion in the DMPK gene. The DMPK RNAs that carry abnormal CUG repeat expansions accumulate in the cell nucleus and lead, in particular, to a loss of function of the MBNL proteins* sequestered by these repeats. His thesis project relates specifically to the loss of function of these proteins in motor neurons and its impact on the maintenance and function of the motor unit.
Interview with Charles Frison-Roche and Frédérique Rau, his thesis co-supervisor.
How does your project fit in with the team’s areas of interest?
Charles Frison-Roche: The team’s work is centred on DM1 and ranges from the instability of the CTG repeats to the development of therapeutic approaches, to understanding the pathophysiological mechanisms. My thesis project fits in with the latter area of interest, and consists of determining the role and the contribution of the loss of function of the MBNL proteins in respect of the motor unit and its impact on the skeletal muscle. More specifically, I am interested in the motor neurons and neuromuscular communication via the neuromuscular junctions (NMJs). To this end, we have developed transgenic mice that no longer express these proteins specifically in their motor neurons. After characterising these mice phenotypically, I examine the molecular alterations found in the motor neurons by conducting a high throughput sequencing of the RNAs, in order to identify the changes caused by the lack of these proteins, both in the spinal cord, where the motor neurons are located, and in the muscle.
What results have you obtained?
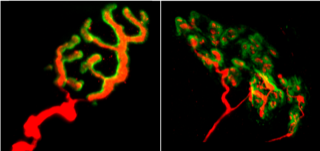
CFR: We have characterised our transgenic mouse model physiologically, which has allowed us to determine that the NMJs are mature but abnormal, with a change in their structure and their function. An alteration in the maintenance of the NMJs could be responsible for this phenotype. We have also observed much larger NMJs in the muscles of our transgenic mice compared to unmodified or wild-type mice. An observation of this phenomenon in biopsies of DM1 patients had, indeed, been reported by Michel Fardeau in his work from a few years ago**.
Physiologically, these transgenic mice models have a locomotion defect and do not walk as well as wild-type mice.
What are the next stages?
CFR: We are now working on the molecular part of the characterisation of our mice, and we will soon be starting to examine the transcriptome using high throughput sequencing of the RNAs. Based on the results obtained, we will test the revealed potential targets in order to determine their contribution to the phenotype observed in our models and in DM1.
Neuromuscular junctions of a healthy mouse (left)
and of a transgenic mouse (right).
Frédérique Rau: It is a general analysis, but the goal is to identify maturation defects for specific RNAs that will impact the function of the corresponding proteins and contribute to the phenotype being observed. The MBNL proteins regulate a huge number of different RNAs, which makes the task complicated. So the aim is to determine which specific RNA(s) could be essential in maintaining the NMJs. We will, therefore, examine all the identified targets and dissect the mechanism of action associated with these defects.
Can these results be used for therapies?
CFR: This is fundamental research; it relates to one of the pathophysiological mechanisms for DM1 and not to the disease in its entirety. However, it will help our team and will pave the way for other therapeutically oriented projects.
FR: This work also raises the question of the accessibility of the therapies: if the motor neuron is, indeed, involved, it will be necessary to consider it as a target tissue on which the therapies will also have to act.
Which aspects of this work were rewarded by the SFM Oral Research Communication Prize?
FR: It was the interest and the quality of the results, and also the clarity of presentation that were rewarded.
The value of this work was also rewarded by the SFM Travel Prize, which offers a bursary to students, so that they can present their work at international conferences.
In 2020, I was able to obtain an ANR Young Researcher award thanks to the preliminary results of Charles’s work.
CFR: These prizes will help me to present my results at the IDMC (International Myotonic Dystrophy Consortium), the largest conference dedicated to DM1, from 21 to 25 June 2022 at Osaka in Japan.
* The MBNL (muscleblind-like) proteins regulate RNA metabolism. In this model that causes them a loss of function, one can observe the consequences resulting from this lack of regulation.
** The origin of this project was electronic microscopy work on the biopsies of patients with DM1 performed by Michel Fardeau (Institute of Myology) in the 1980s. He observed that the NMJs in DM1 were abnormally large, a characteristic that is not found in the other pathologies where this structure is impacted.
