Bruno Cadot, researcher at the Center of Research in Myology and head of the MyoImage technology platform, oversaw the acquisition of a latest-generation Nikon AXR-NSPARC confocal microscope equipped with an N-STORM super-resolution module*. This new high-performance device adds to the platform’s range of cutting-edge imaging and image analysis equipment. Interview with Bruno Cadot
What are the features of your new confocal microscope?
A fluorescence microscope uses the emission properties of a specific wavelength of certain proteins or fluorophores when excited by a shorter wavelength. Compared to a conventional wide-field fluorescence microscope, a confocal fluorescence microscope eliminates light emitted outside the focal plane of the observed area, thereby producing very high-quality images. In general, the emitted light is collected by a detector that converts the quantity of photons received into an image. The solution acquired uses 25 detectors, which allows for an even finer and higher-contrast image to be obtained in order to better characterize biological structures.
However, due to the physical properties of light, fluorescence microscopy is limited to separating elements larger than 200 nanometres (nm), meaning that if two objects are less than 200 nm apart, they cannot be distinguished and only one object will be visible. However, many biological structures measure less than 200 nm, and the only possible alternative for observing them is currently to use an electron microscope. With a resolution of up to 5 nm, however, it only allows structures to be observed in greyscale, unlike fluorescence microscopy, which allows the different proteins chosen for observation to be seen by colouring or marking them. I therefore decided to add a STORM super-resolution module to this new confocal microscope in order to overcome this resolution barrier and achieve 20 or 30 nm.
How does it work?
To use the STORM module, which stands for Stochastic Optical Reconstruction Microscopy, we use fluorophores that are capable of flashing. We then record a series of images of the different fluorophores flashing randomly (stochastically), a bit like the Eiffel Tower at night. Each flash corresponds to several pixels on the image, but only the centre of this cloud is retained. By accumulating between 100 and 1,000 images, we are able to reconstruct a hyper-resolved image, thereby lowering the resolution limit to around 20 nm!
What projects will you use it for?
I work on the nuclear envelope, so this microscope will enable me to better understand how the network of lamins is organised there, what interactions occur, and what impact mutations have on this organisation in healthy and diseased muscle.
All of the Institute’s research teams will use it, because today, the confocal microscope has become the ‘routine’ microscope for all projects. For example, whether in DM1 due to RNA aggregates or in ALS, which presents aggregates in neuronal cells, it is currently almost impossible to discern the distribution and organisation of their components. This new super-resolution technology will shed new light on both the understanding of these diseases and other projects studying subcellular structures.
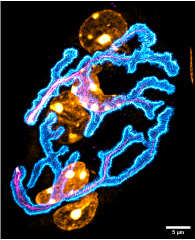
Fig.1. Image acquired using the N-SPARC confocal microscope
Could this microscope be used for more than just fundamental research?
STORM could also become a diagnostic tool for clinicians. Although this is not the primary objective at present, it would be possible to use the key elements observed at these levels of resolution to better understand and characterise pathologies.
By making a more accurate diagnosis at the cellular level, i.e. at a very early stage, it would be possible to intervene at an early stage, before more significant effects occur on the entire muscle.
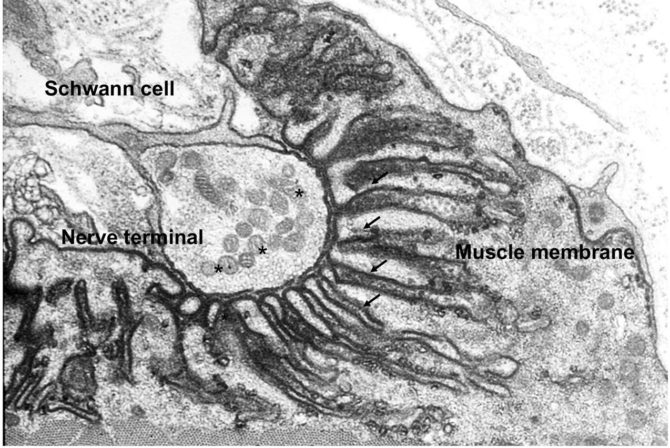
Fig.2. Image acquired by electron microscopy
* The acquisition of this new, latest-generation confocal microscope was made possible in part thanks to significant funding from our partner Peugeot Invest.
Figures
The neuromuscular junction is a structure that transmits contraction stimuli from the brain to nerve endings and muscle fibres. The interface between these two cell types is crucial, and its structure determines whether the situation is healthy or pathological.Fig. 1. Image acquired using N-SPARC confocal fluorescence microscopy for the acetylcholine receptor (cyan), nerve (magenta) and nuclei (yellow). Details of the distribution of receptors can be seen, in particular the gutters, small structures corresponding to invaginations of the plasma membrane. Out-of-plane structures are not visible. © Bruno Cadot
Fig. 2. Image acquired by electron microscopy showing the nerve ending at the muscle fibre membrane. The gutters or invaginations of the plasma membrane are visible (arrows). © NMJ Frontiers