
Fig.1
Bruno Cadot, a researcher in Marc Bitoun’s team at the Myology Centre for Research, has successfully acquired a new confocal microscope for the institute. Eighteen months elapsed between the first applications for funding and installation. Since its inauguration last month, researchers and clinicians have been lining up to use it.
What happened during those 18 months?
In July 2016, DIM Biothérapies launched a call for projects in the Ile de France region, which subsidises up to 66% of the cost for the purchase of equipment. The remaining 33% was funded by AIM. Between the response to the call for projects, the successive phases of project selection, searching for additional financing, testing microscopes that meet the criteria selected by the various suppliers, and ordering the chosen device and its installation, 18 months had passed.
Why was it necessary to acquire such an equipment?
Even though the Institute has very good electron microscopes (EMs), a confocal fluorescence microscope was missing because, unlike EMs, living cells can be observed. For this type of observation, previously the teams of the Institute had to rent confocal microscopes from a nearby technical platform. Our new microscope combines the latest and most advanced technologies: better resolution, considerably reduced exposure time for capturing images, a wider visual field, the possibility of filming movements on living material (proteins inside cells, cells in a tissue, and even small, whole organisms like the zebrafish larva). After one or two hours of training, using the microscope is simple and fast and it is accessible to all of the teams.
 How are you going to use the microscope?
How are you going to use the microscope?
I will follow the deformation of nuclear envelopes and thus differentiate the proteins located inside and outside this envelope, observe whether they interact and how they modify their position within the nuclear envelope during the migration of nuclei, for example.
But it is also possible to reconstruct a 3D image of the internal organisation of a muscle fibre and the organisation between fibres. One can visualise in 3D, how protein aggregates are positioned within the nucleus, where they are located, their size etc. We now have access to a more significant wealth of information than we previously had.
It is also possible to perform image mosaics to carry out statistical analyses of various measures: size, shape, distance between different structures… If living cells are observed “live”, it is possible to evaluate their movement, speed, when they come into contact, fusions, separations etc. In fact, the possibilities are infinite!
Fig.1. The microscope is a Nikon Ti2 coupled to a Yokogawa CSU-W1 head, a super-resolution Live-SR module from Gataca Systems and equipped with two cameras; a Prime 95B from Photometrics and a DS-Ri2 from Nikon. Temperature maintenance and CO2 is achieved by a device from Oko-Lab.
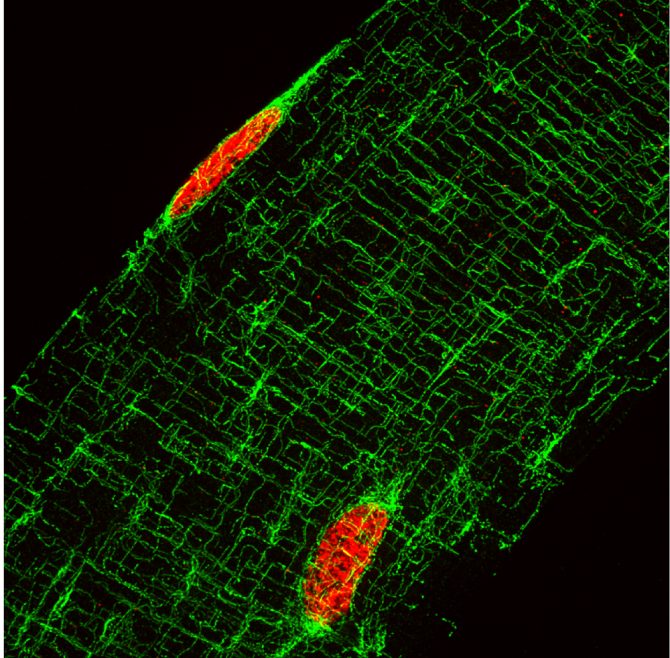
Fig.2. Projection of 35 images, 3.5um total thickness, image of an isolated muscle fibre, with microtubules labelled in green and nuclei in red. One can clearly see the highly organised network of this cytoskeleton.
