Since March 2015, Fabien Le Grand has led the group “Regeneration, physiology and therapies” in Vincent Mouly’s team at the Myology Centre for Research.
Since March 2015, Fabien Le Grand has led the group “Regeneration, physiology and therapies” in Vincent Mouly’s team at the Myology Centre for Research.
He and his team have published an article* in Cell Reports concerning Wnt/b-catenin activation in adult muscle progenitor cells.
What was the objective of this study?
It is well established that the canonical Wnt signals (that activate the b-catenin dependent pathway) are activated during tissue regeneration and overexpressed in DMD, FSH and ageing muscles.
The study that has just been published was carried out to understand the role of Wnt/b-catenin activation in muscle stem cells (MuSCs) and adult muscle progenitor cells.
What have you concluded?
Our conclusion is that neither too much nor too little activation (Goldilocks principle) is essential! Following an injury, quiescent MuSCs are activated and proliferate to give rise to muscle progenitor cells. In these cells, Wnt/b-catenin signalling becomes activated and precisely controls the balance between proliferation and differentiation: it should be “correctly” regulated in order for muscle tissue to regenerate after an injury.
How did you demonstrate this?
For this, we generated conditional genetic models to modulate the Wnt/b-catenin pathway only in MuSCs and we analysed muscle regeneration in these mutant mice. If we genetically activate b-catenin in progenitor cells derived from MuSCs, they differentiate too quickly and the muscle regenerates poorly.
Indeed, the cells do not have enough time to proliferate correctly and fuse too quickly. Therefore, the regenerated muscle is composed of small fibres and the tissue shows signs of premature aging. Conversely, if the Wnt signaling pathway is inhibited by disrupting the gene that codes for b-catenin, muscle progenitor cells derived from MuSCs differentiate less efficiently, which also leads to poor muscle regeneration. We conclude that the Wnt signalling pathway influences cell fate by temporally controlling the proliferation and differentiation of MuSCs.
What are the next steps?
We will use small molecules that target the Wnt signaling pathway in Duchenne muscular dystrophy mouse models: this will improve the condition of the affected muscle by activating endogenous regeneration and reducing fibrosis. Thus, by improving the condition of a patient’s muscle, it will be prepared to receive a gene therapy treatment since one of the biggest obstacles to this type of therapy is precisely the condition of the patient’s muscle.
Specifically, we will work with Stéphanie Lorain (team 5 at the Centre for Research) who works on the maintenance of AAV genomes in dystrophic muscles. Indeed, when the fibre is damaged (fibrosis, inflammation, etc.), the vector is not retained and expression of the therapeutic vector gradually disappears. So we will try to downregulate activation of the pathway by testing, for example, porcupine inhibitors, the protein responsible for Wnt secretion (already used in oncology). Once we have obtained the desired effect in the mouse model, we will perform studies on cells from patients with Duchenne muscular dystrophy and on immortalised cell lines from Vincent Mouly’s platform. Our goal is to restore the “correct level” of Wnt/b-catenin in dystrophic cells to enhance their healing potential.
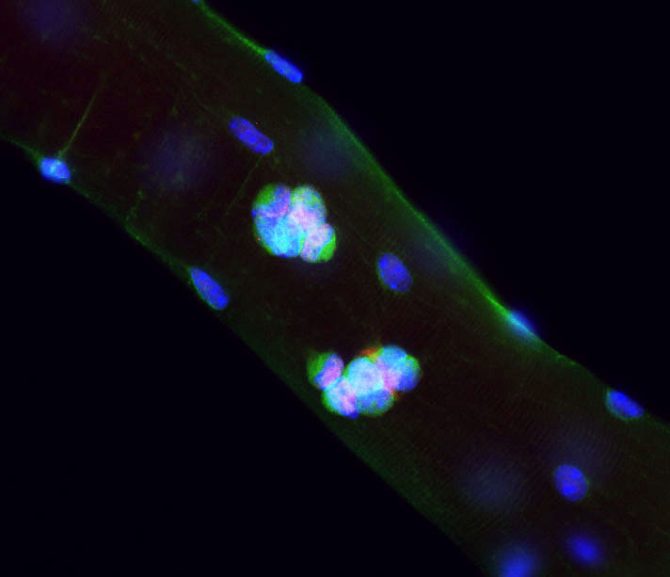
Figure (1) Myofibre
Muscle stem cells located around myofibres can become activated and give rise to clones of muscle progenitor cells. Here, an intact myofibre was isolated and cultured for 3 days. Progenitors derived from stem cells express the transcription factor Pax7 (red) and the surface protein Vangl2 (green).
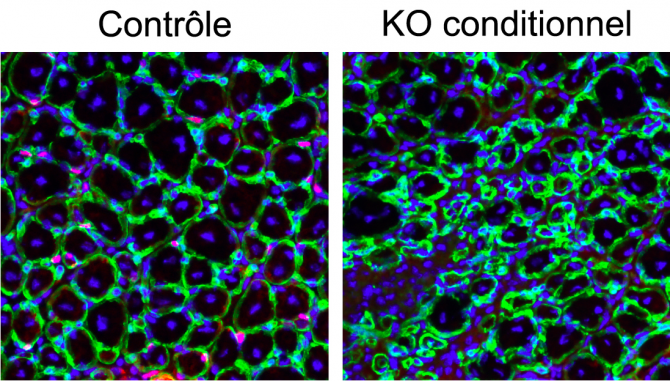
Figure (2) Regeneration
Muscle tissue regeneration was induced by injection of cardiotoxin. After 7 days, the muscles were removed and cross-sections were collected. In the control, muscle progenitor cells were able to regenerate new myofibers surrounded by a basal lamina (green) with central nuclei (blue). However, when the expression of beta-catenin is genetically inhibited in muscle stem cells, the muscle does not regenerate normally.