
Satish Babu Moparthi, post-doctoral fellow, and Stéphane Vassilopoulos, co-director of the ‘Muscle cell organization and therapy of dominant centronuclear myopathy‘ team at the Research Centre of the Institute of Myology, have just published an article in the journal Science* in collaboration with Christophe Leterrier’s team (Aix-Marseille University, CNRS). Using a combination of two cutting-edge technologies, they have revealed a hitherto unknown mechanism of endocytosis in the axon. – Interview with Stéphane Vassilopoulos
How did your collaboration with Christophe Leterrier come about?
The story began in 2016 when I contacted Christophe Leterrier, a neurobiology researcher working on the ultrastructure of neurons using the STORM super-resolution (SR) photonic microscopy technique (which won its inventors the Nobel Prize for Chemistry in 2014). At the time, I had managed to strip neurons using the metal replica technique that we use in our laboratory**, and it was so beautiful and unexpected to see neurons in this way that I contacted Christophe to send him these photos and suggest that we work together. We decided to work together on an extremely specific project: to visualise the actin rings regularly spaced along the axon membrane. Until then, it had only been possible to see them in SR but never in high-resolution electron microscopy, which, for some researchers, cast doubt on their existence and was more an artefact linked to the reconstruction of the super-resolution image.
How did you go about it?
Christophe first stripped the neurons in Marseille and imaged them using SR STORM, then sent them to me in Paris where I imaged them using electron microscopy. It was magnificent: in the neuron, the actin formed rays, precisely 180 nm apart, which looked like little scales. It was the first time we had seen them at this resolution.
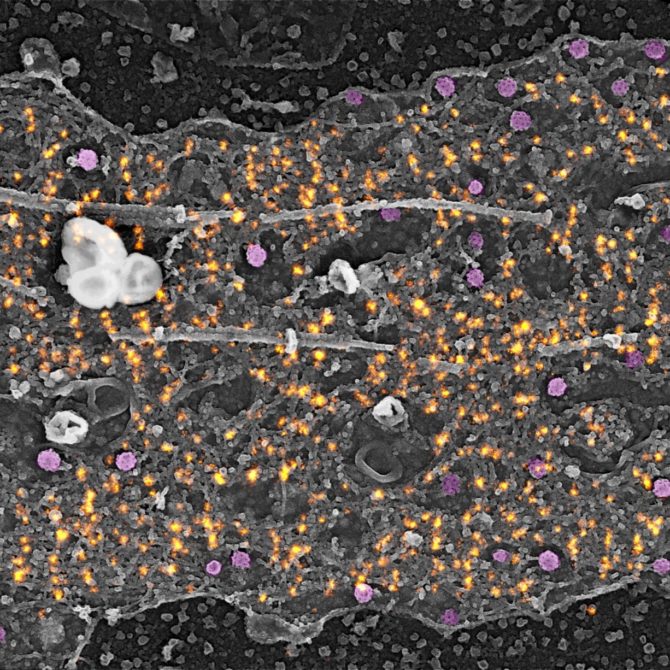 We then took the work further (and it’s unique in the world!) and first of all he took images of the neurons in SR in Marseille: he stripped the neurons, marked the actin, imaged it on a single neuron on which he put a marker so that I could find it, to observe this same neuron from the electron microscopy. We then superimposed his image of actin on a nanometric scale in SR and my image of the neuron in electron microscopy, showing its structure on the same scale [photo ?]. This work was published in Nature Communications** in December 2019.
We then took the work further (and it’s unique in the world!) and first of all he took images of the neurons in SR in Marseille: he stripped the neurons, marked the actin, imaged it on a single neuron on which he put a marker so that I could find it, to observe this same neuron from the electron microscopy. We then superimposed his image of actin on a nanometric scale in SR and my image of the neuron in electron microscopy, showing its structure on the same scale [photo ?]. This work was published in Nature Communications** in December 2019.
What about the work recently published in Science?
It was by observing the actin rings in the axon that we discovered that there were clathrin pits along the axon, whereas it had previously been accepted that in the neuron there were only pits at the synapses and in the cell body. What’s more, these clathrin-covered pits were not classical but only present in structures that we called ‘clearing’, formed by an interruption in the submembrane meshwork of spectrin, which protects the axon membrane. These clearings allow clathrin access to the membrane for endocytosis.
However, under basal conditions, we did not observe endocytosis at this level, which is ultra-stable. There is competition between spectrin and clathrin, and the meshwork prevents endocytosis. To demonstrate endocytosis, we used a chemical molecule (NMDA) that induces strong neuronal activity, which activates endocytosis. In other words, when the neuron begins to function and transmit electrical currents, endocytosis rapidly takes place to modulate the proteins present on the neuron’s surface. This ability of the neuron to modify its functional and structural properties is known as neuronal plasticity. In fact, the vesicles are immobile, waiting for a ready-to-go signal that occurs when there is intense stimulation of the neuron, triggering endocytosis.
Finally, we revisited the whole concept of endocytosis in the axon to show that endocytosis does occur and that it is not constitutive but finely regulated by the activity of the neuron.
Did you call on the Myology Institute’s technological platforms**** and if so, at what level of the work?
Yes, we did call on the expertise of the Institute’s teams. In particular, the MyoVector platform, headed by Sofia Benkhelifa-Ziyyat, enabled us to make AAV vectors to break down spectrins in neurons. Gilles Moulay, who runs the MyoMolBio platform, designed and produced the shRNA tools (used to break down a sequence) that were integrated into the vectors. Jeanne Lainé, an expert in electron microscopy, has also worked extensively on neurons.
What prospects does this work open up?
From a technological point of view, this discovery of the possibility of combining photonic and electron microscopy to observe neurons is unique in the world and, as well as producing magnificent images, offers the opportunity to observe, compare and visualise phenomena on a nanometric scale to better decipher them. We have combined two exceptional techniques to create added value that goes beyond the sum of the two!
From a scientific point of view, this discovery has enabled us to understand a mechanism that could be defective in myopathies. In muscle cells, clathrin has different properties and forms plaques rather than vesicles as it does in the axon. This being the case, understanding the interaction between spectrins and clathrin at the neuronal level opens up new avenues for understanding neuromuscular pathologies and opens up new possibilities for therapeutic intervention.
________
** Our laboratory has developed an electron microscopy technique for visualising proteins on the inner surface of the cell. The process involves breaking up the cell using ultrasound, then creating a replica of the surface using platinum grains so that it can be observed with the electron microscope. The nanometric resolution enables the structures formed by the proteins to be observed in their entirety.
**** https://www.institut-myologie.org/en/expertises/platforms-technology-units/
